(Natural News)
On the latest episode of “Doctors and Scientists,” Dr. Brian Hooker Ph.D., P.E., was interviewed Dr. Jessica Rose, Ph.D. to discuss the failures of the vaccine injury surveillance system that was set up by the CDC and FDA over thirty years ago. Dr. Rose is an expert in bio-mathematics and molecular research.
In January of 2021, she utilized her skills as a computational biologist and began analyzing data in the Vaccine Adverse Events Reporting System (VAERS). Each week, she downloaded publicly-available data sets from VAERS, comparing inputs week-to-week. She discovered that vaccine injury reports went missing from one week to the next. Each week, the data is updated in the VAERS system. She found that some of the data is overwritten, vanished from the system. She also found that “hundreds of thousands” of covid vaccine injury reports were backlogged and did not appear in a timely manner to alert healthcare professionals to serious issues with the vaccine.
Hundreds of thousands of vaccine injury reports backlogged in VAERS
In the interview, Dr. Rose discussed the systemic flaws of the VAERS system, flaws that stop the passive reporting system from working in the public’s interest, as was originally intended. The pharmacovigilance system was set up in 1990 to detect issues with vaccines, to alert regulatory agencies and the public about serious adverse events and contraindications for specific vaccines. The data is managed by the Department of Health and Human Services. Healthcare professionals input the data into the system, and have a narrow thirty-minute window to complete the report. In 2021, healthcare workers have been overwhelmed with vaccine injury reports and have not had the time to enter them all into the system. Many medical concerns associated with the covid vaccine are overlooked, discarded or discounted as coincidental or normalized reactions to the vaccine.
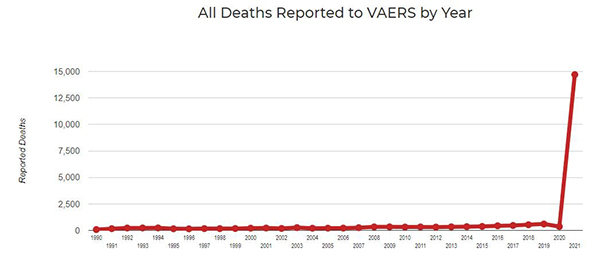
The hundreds of thousands of adverse event reports that have been filed paint a grisly picture of medical malfeasance. These serious public health issues have yet to be addressed by any regulatory agency or judicial process. In the past, vaccines were pulled from the market if the VAERS system documented more than fifty deaths from a single vaccine. In 2021, there have been more than 20,000 deaths recorded in just ten months. Up to 97 percent of these issues are coming from the new mRNA covid vaccines, not the rest of the vaccine supply. Instead of pulling the deadly products from the market, the federal government has issued unlawful mandates, coercing individuals to take part in the depopulation experiment. This might be the biggest flaw with the system yet: The agencies that are supposed to oversee the data and alert the public to medical atrocities are the same entities trying to push a narrative forward – that vaccines are “safe and effective.” (Related: COVID vaccine experiment causes monstrous spike in vaccine injuries and deaths, serious adverse events under-reported by a factor of eight.)
Serious adverse events and fatalities are occurring at magnitudes greater than what is recorded in the VAERS system
After analyzing missing data in the VAERS system, Dr. Rose came to the conclusion that serious adverse events and fatalities following covid vaccination are much higher than what is recorded in the VAERS system. Some issues are under-reported by a factor of thirty-one, and other, more common side effects can be under-reported by a factor of one hundred. By September, Dr. Rose attended the FDA’s Vaccine and Related Biological Products Advisory Committee meeting, bringing attention to under-reporting problem in the VAERS system. Her research is titled, “Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Event Reporting System (VAERS) a Functioning Pharmacovigilance System?” and was published in Science, Public Health Policy and Law.
Her paper concludes that “hundreds of thousands” of adverse events are backlogged and waiting to be entered into the system. “The most important thing I found in my determination is whether or not this tool — which can be a pharmacovigilance tool — is being used as such,” Dr. Rose said.
Sources include:
IPAKPHPI.com [PDF]